The Substance Clo3-1 Is Best Described as
The correct name for FeO is. Calculate the standard cell emf for the reaction.
D distorted tetrahedron seesaw.
. What is the correct formula for sodium carbonate. 1 See answer Advertisement Advertisement mayzeeabostick is waiting for your help. It is soluble in waterThe material itself is noncombustible but it can form a very flammable mixture with combustible materials and this mixture may be explosive if the combustible material is very finely divided.
A bond between a metal and a nonmetal. Now draw a second structure that is. The substance ClO3- is best described as.
Bonds Lone pairs c. The carbonate ion has the formula CO32-. Main concepts - Lewis Structures a.
A the sharing of electrons between atoms. The substance ClO 3- is best described as a. CO32-the correct formula for the carbonate ion is.
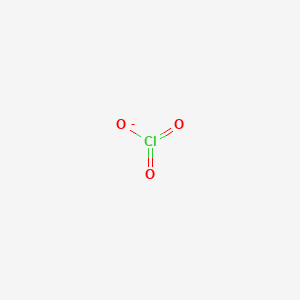
Charged entity composed of 2 or more different elements. Therefore the name of HClO 3 is. The chlorate ion ClO3- is trigonal pyramidal and polar.
The transfer of electrons. Here is a site that will show you how to determine the oxidation. I just to know how to identify the substance that is reduced the substance that is oxidized the oxidizing agent and the reducing agent in each reaction.
The substance ClO3-1 is best described as. ClO3 is the chemical formula of chlorine trioxide more correct is Cl2O6. B 1 lone pair bent E 3 lone pairs linear C 2 lone pairs bent Ans.
A molecule Question 22. E145 V Express the emf to three. The gain of electrons is reduction.
Cl O O O. The correct formula of chlorate ion is ClO 3-. The substance ClO3 is best described as.
Please help with questions 11-13. You look to see which elements change oxidation state. Up to 256 cash back Make sure you answer all questions.
Draw a Lewis structure for the chlorate ion ClO 3-in which all atoms satisfy but do not exceed the octet rule. All other atoms do not have charges. Is a white crystalline.
Since for the molecule to be polar there must be 1High electronegativity difference between the respective atoms 2. Lewis Dot of the Chlorate Ion ClO3. The chemical name for ClO3 is chlorate ion.
Calculate the standard emf using data in Appendix E and given the following. A covalent bond is best described as. The geometry of the CS 2 molecule is best described as A linear.
The correct name for an aqueous solution of HBr is. According to the VSEPR theory the geometry of the SO 3 molecule is A pyramidal. Practice test 3 Student.
The fundamental unit of volume in the SI system is the. The anion ClO3- is the chlorate. Which of the following is a binary compound.
MathrmSCl_2 quad text b. The chemical bond formed by the electrostatic. The chemical name for ClO 3- is chlorate ion.
What is A for ClO3 the chlorate ion. Electron Geometry Molecular Shape d. A polyatomic ion d.
View Test Prep - Test 3 Practice from CHM 1045 at Florida International University. A polyatomic molecule C. Only one oxygen atom has a -1 charge.
When you name ionic compounds with metal ions that can have more than 1 charge you use a. PCl4 NO3- O3 trans - CCl2F2 are non polar. Add your answer and earn points.
There is no molecule or ion as ClO3. Resonance Structures Formal Charge FC is a charge an atom in the molecule or ion would have if all bonding electrons were shared equally between the bonded atomsLewis structure of ClO3- ion. I know correct answer is E-Chloric acid but I need explanation.
Identify the substance with ionic bonds. The loss of electrons is oxidation. The chlorate ion has a 1-.
B c d a bond between a metal and a polyatomic ion c a bond between two polyatomic ions. New questions in Chemistry. A polyatomic ion D.
The answer is 26 I have the same course. It is soluble in water. The substance ClO3- is best described as.
ClO3aq3Cus6HaqClaq3Cu2aq3H2Ol Pt is used as an inert electrode in contact with the ClO3 and Cl. A Ne b CO d H20e. Therefore overall charge of chlorate ion is -1.

Clo3 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
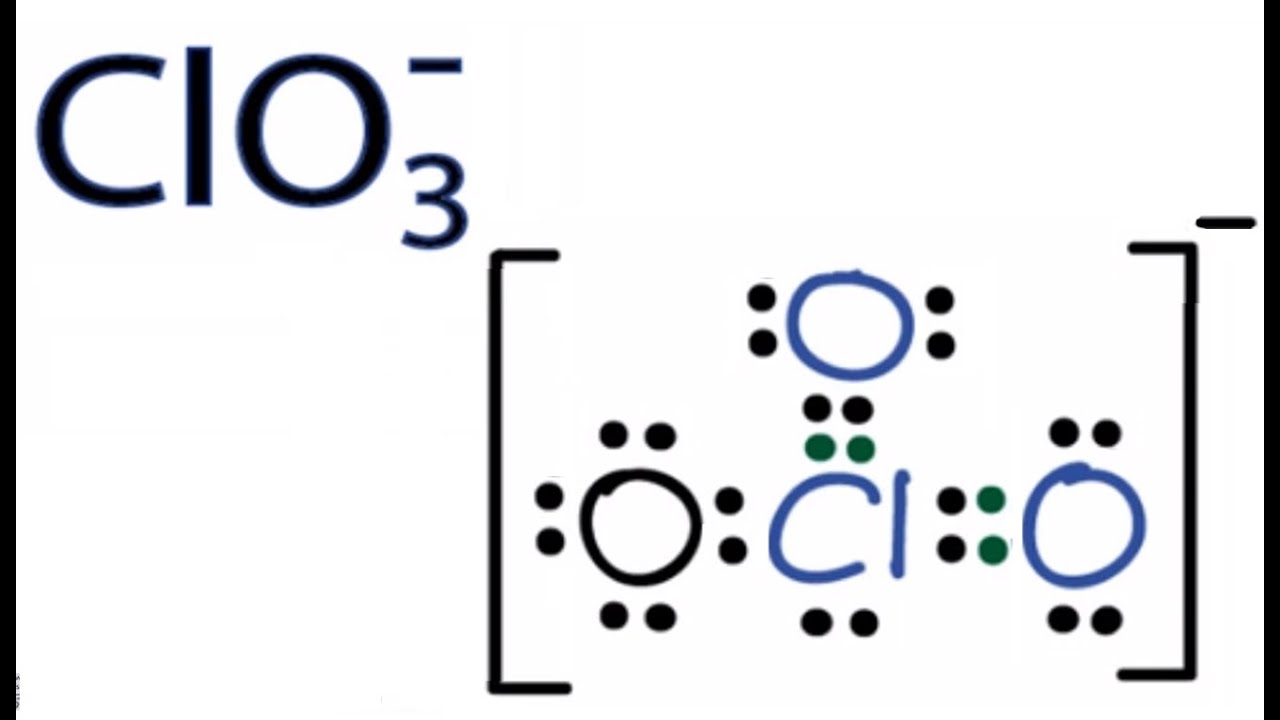
Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube
Comments
Post a Comment